Abstract
Introduction: The hypomethylating agents (HMA) are the standard of care for a majority of patients with higher-risk MDS. SGI-110 is a second generation HMA that molecularly is a dinucleotide derivative of decitabine and therefore a more potent inhibitor of DNA methyltransferase activity. SGI-110 is currently being studied in front-line AML and second-line MDS multicenter studies. Here we present results of a single arm phase II trial of SGI-110 for patients with previously untreated MDS.
Methods: Patients, age 18 or older, with adequate renal and hepatic functions, with int-2 or high risk MDS by IPSS or more than 10% blasts in bone marrow were eligible. One prior cycle of azacitidine or decitabine was allowed. No prior other therapies were allowed. SGI-110 was administered at a dose of 60 mg/m2 SC daily x 5 days every 4 weeks. The study was designed with stopping rules for response, toxicity, and mortality (first 3 months). A maximum of 100 patients could be treated.
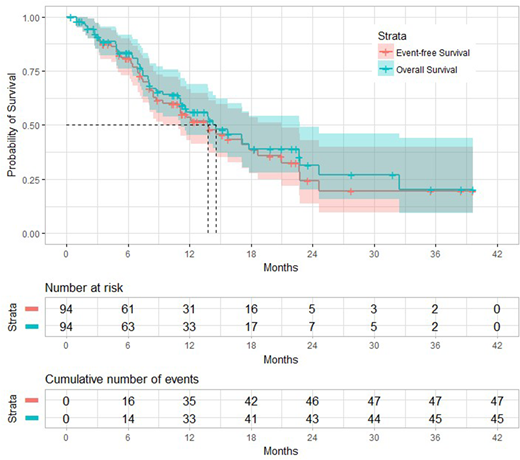
Results: From 11/14/2014 to 7/31/2018, 94 patients have been treated. Median age was 69 years (22.7-91.9), 72 patients (77%) had INT-2, 13 patients (14%) high risk. Median % of marrow blasts was 10 (range, 0-20). Median white blood cell count and platelet count were 2.5 (×106/L), and 52 (×106/L) respectively. Twenty two patients (23%) were diploid, 36 (38%) complex, and 33 (35%) others. Mutation distribution was as follows: TP53, 29 (31%); ASXL1, 26 (28%); TET2, 20 (21%); RUNX1, 19 (20%); RAS, 12 (13%); DNMT3A, 10 (11%); EZH2, 9 (10%); SRSF2, 6 (7%); PHF6, 4 (4%); BCOR, 3 (3%); CEBPA, 3 (3%); SF3B1, 3 (3%); IDH2, 3 (3%); BRAF, 2 (2%); CBL 2 (2%); MPL, 2 (2%); NPM1, 2 (2%); U2AF1, 2 (2%); WT1, 2 (2%); CREBBP, 1 (1%); ETV6, 1 (1%); FLT3-ITD, 1 (1%); GATA2, 1 (1%); IDH1, 1 (1%); SETBP1, 1 (1%); ZRSR2, 1 (1%). The median number of cycles received was 5 (range 1 - 32). Ninety four (100 %) patients are evaluable for toxicity. Early mortality was 0%. Common toxicities were fatigue (61%), infection (46%), nausea (27%), pain (19%), and constipation (16%), mucositis (16%), dyspnea (15%), local injection toxicity (15%), and diarrhea (12%). Eighty seven (93%) patients were evaluable for response. The median number of cycles to response was 3 (range 1 - 11). Overall response rate was 53 (61%); CR 19 (22%), CRp 3 (3%), HI 31 (36%), SD 5 (6%), NR 27 (31%), and died 2 (2%). With a median follow-up of 15 months, the median OS was 15 months and the median EFS was 14 months (Figure 1). By UVA, higher ACE-27 score showed tendency of lower rates of response (p=0.063; hazard ratio [HR], 1.383; 95% confidence interval [CI], 0.982-1946). However, MVA did not show any prognostic factors for response. By MVA characteristics associated with survival were: complex karyotype (p=0.036; HR, 2.345; 95% CI, 1.055-5.210), and response to therapy (p=0.003; HR, 0.272; 95% CI, 0.114-0.648).
In conclusion: SGI-110 is well tolerated in previously untreated MDS. ORR appears to be better than expected compared to azacitidine or decitabine. Longer follow-up and randomized trials will be needed to understand effect on survival.
Sasaki:Otsuka Pharmaceutical: Honoraria. Bose:Incyte Corporation: Honoraria, Research Funding; CTI BioPharma: Research Funding; Celgene Corporation: Honoraria, Research Funding; Astellas Pharmaceuticals: Research Funding; Constellation Pharmaceuticals: Research Funding; Blueprint Medicines Corporation: Research Funding; Pfizer, Inc.: Research Funding. Daver:Pfizer: Consultancy; Karyopharm: Research Funding; Novartis: Consultancy; Daiichi-Sankyo: Research Funding; Karyopharm: Consultancy; ARIAD: Research Funding; Novartis: Research Funding; Incyte: Research Funding; Incyte: Consultancy; BMS: Research Funding; Otsuka: Consultancy; Alexion: Consultancy; Sunesis: Consultancy; Pfizer: Research Funding; Sunesis: Research Funding; ImmunoGen: Consultancy; Kiromic: Research Funding. Ravandi:Bristol-Myers Squibb: Research Funding; Sunesis: Honoraria; Orsenix: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Astellas Pharmaceuticals: Consultancy, Honoraria; Xencor: Research Funding; Seattle Genetics: Research Funding; Abbvie: Research Funding; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Bristol-Myers Squibb: Research Funding; Jazz: Honoraria; Seattle Genetics: Research Funding; Abbvie: Research Funding; Jazz: Honoraria; Sunesis: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Macrogenix: Honoraria, Research Funding; Macrogenix: Honoraria, Research Funding; Xencor: Research Funding. Cortes:Pfizer: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Arog: Research Funding. DiNardo:Celgene: Honoraria; Agios: Consultancy; Karyopharm: Honoraria; Abbvie: Honoraria; Bayer: Honoraria; Medimmune: Honoraria. Pemmaraju:SagerStrong Foundation: Research Funding; Affymetrix: Research Funding; plexxikon: Research Funding; daiichi sankyo: Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; abbvie: Research Funding; cellectis: Research Funding; stemline: Consultancy, Honoraria, Research Funding; novartis: Research Funding. Kadia:Novartis: Consultancy; Amgen: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; Abbvie: Consultancy; Pfizer: Consultancy, Research Funding; BMS: Research Funding; Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Celgene: Research Funding; BMS: Research Funding; Celgene: Research Funding; Novartis: Consultancy; Jazz: Consultancy, Research Funding; Abbvie: Consultancy; Takeda: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal